Introduction: BCMA-directed chimeric antigen receptor T-cell therapy (CAR T), idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel), are approved for patients with relapsed and/or refractory (R/R) multiple myeloma (MM). Pivotal KaRMMA and CARTITUDE-1 trials as well as real-world data demonstrated heterogeneity in toxicity and efficacy between ide-cel and cilta-cel. To account for such differences, we have established a novel composite endpoint of toxicity-free, progression-free survival within 3 months (TPFS3) after anti-BCMA CAR T therapy. We also studied factors associated with TPFS3 across ide-cel and cilta-cel and the impact of TPFS3 on overall survival (OS) after CAR T.
Methods: We analyzed a retrospective cohort of 133 consecutive recipients of ide-cel or cilta-cel at Moffitt Cancer Center (05/2021-03/2023). TPFS3 was defined as absence of severe (Grade≥3) cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), cytopenia(s), myeloma progression/stable disease (PD/SD), and non-relapse mortality (NRM) within 3 months after CAR-T infusion. CRS and ICANS were graded according to ASTCT Consensus grading. Cytopenias were graded according to CTCAE v5.0 and defined as two (or more) timepoint uni- or multilineage cytopenia, including any Grade≥2 cytopenia at post-CAR T Day 30 deepening to at least Grade≥3 at Day 60 and/or Day 90. Factors associated with TPFS3 were assessed via multivariate logistic regression analysis (MVA). Landmark Cox survival analysis at the 3-month timepoint examined the impact of TPFS3 on subsequent overall survival (OS). All analyses were performed using SAS and R.
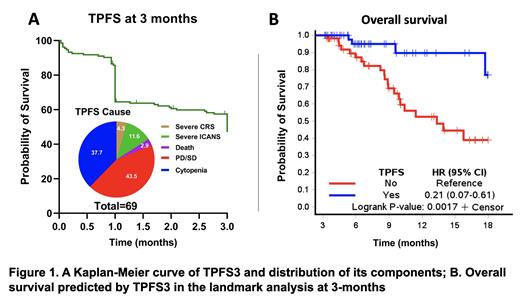
Results: In total, 48.1% (N= 64; median age=66 years [range, 45-82]; male=44%) of the patients were alive, toxicity- and progression-free by 3 months after CAR T infusion. TPFS3 components included PD/SD (N=30, 43.5%), severe cytopenias (N=26, 37.7%), ICANS (N=8, 11.6%), CRS (N=3, 4.3%), and NRM (N=2, 2.9%) (Figure 1A). In the univariate analysis, TPFS3 patients differed from the rest of the cohort according to lower marrow burden ( p=0.025), revised international staging system (R-ISS) (I/II vs III; p=0.015), CRP ( p=0.019) and ferritin ( p=0.002) levels at lymphodepletion (LD). The use of ide-cel (N=109) vs cilta-cel (N=24) was not significantly associated with TPFS3 ( p=0.119).
In the MVA logistic regression, lower ferritin level (median cut off=228 ng/mL) at LD was associated with TPFS3 (odds ratio [OR]=0.37 [95%CI, 0.18-0.78], p=0.008] after adjusting for age, myeloma marrow burden, presence of extramedullary disease, R-ISS, CAR T product (all p>0.1), and CRP (median cut off=0.41mg/dL) at LD (OR=0.51 for lower vs higher, [95%CI, 0.25-1.05], p=0.069) included in the final model together with ferritin. In the landmark Cox survival analysis at 3 months, TPFS3 was associated with 6.54-fold superior OS (hazard ratio [HR]=0.15, 95%CI 0.05-0.52, p=0.003) (Figure 1B) after adjustment for potential confounders (age, gender, CRP, CAR-T product, marrow burden, R-ISS, extramedullary disease; all p>0.1). Other prognostic risks associated with inferior OS in the final model of the landmark analysis included the use of bridging therapy (HR=3.98 [95%CI, 1.30-12.20], p=0.016), ECOG≥2 at LD (HR=4.53 [95%CI, 1.57-13.07], p=0.005) and higher ferritin level at LD (HR=2.60 [95%CI, 0.95-7.13], p=0.063).
Conclusions: We found that 48.1% of anti-BCMA CAR T recipients with R/R MM were alive and free of TPFS3-defining events. Myeloma response failure, cytopenias and severe ICANS were the top leading components of TPFS3 which was best predicted by ferritin level at LD. TPFS3 independently predicted long-term OS. Thus, TPFS3 may serve as an ideal composite safety and efficacy endpoint in future myeloma trials with BCMA-directed CAR T.
Disclosures
Nishihori:Medexus: Speakers Bureau; Moffitt Cancer Center: Other: Personal fees from Karyopharm and Novartis outside the submitted work. Liu:BioLineRx: Membership on an entity's Board of Directors or advisory committees. Jain:Loxo@Lilly: Research Funding; Incyte: Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Myeloid Therapeutics: Consultancy, Honoraria. Blue:Sanofi: Consultancy; Oncopeptides: Consultancy; AbbVie: Consultancy; Pfizer: Consultancy; Kite Pharmaceuticals: Consultancy; Janssen: Consultancy. Grajales-Cruz:Amgen: Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cellectar: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Castaneda:Adaptive Biotechnologies: Speakers Bureau; Moffitt Cancer Center: Current Employment. Shain:Amgen: Honoraria; Amgen: Honoraria; Bristol Myers Squibb: Honoraria; Karyopharm: Research Funding; Janssen: Honoraria; Adaptive: Honoraria; Sanofi: Honoraria; GlaxoSmithKline: Honoraria; Takeda: Honoraria; AbbVie: Research Funding. Baz:Regeneron: Research Funding; GSK: Honoraria; HIKMA Cancer Network: Honoraria; Curio Science: Honoraria; AbbVie: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AHOMPR: Honoraria; ASH: Honoraria. Freeman:Janssen: Consultancy, Honoraria, Research Funding; ONK Therapeutics: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Alsina:Genzyme: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Research Funding; Janssen Oncology: Consultancy, Speakers Bureau; RevHealth LLC, Red Med LLC: Honoraria. Locke:GammaDelta Therapeutics: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Cowen: Consultancy; Cellular Medicine Group: Consultancy; Caribou: Consultancy; EcoR1: Consultancy; Legend Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Other: Travel Support; Daiichi Sankyo: Consultancy; Umoja: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioPharma Communications CARE Education: Other: Institutional; Pfizer: Membership on an entity's Board of Directors or advisory committees; Sana: Consultancy, Membership on an entity's Board of Directors or advisory committees; Wugen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Society for Immunotherapy of Cancer: Other; ASH: Other: Travel Support; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Emerging Therapy Solutions: Consultancy, Other; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional ; Calibr: Consultancy; Clinical Care Options Oncology: Other; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imedex: Other; CERo Therapeutics: Other: (Institutional); Iovance: Consultancy, Membership on an entity's Board of Directors or advisory committees; Leukemia and Lymphoma Society: Other; National Cancer Institute: Other; Gerson Lehrman Group (GLG): Consultancy; Bristol Myers Squibb/ Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; A2 Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Hansen:Pentecost Family Myeloma Research Center: Research Funding; Janssen: Consultancy; International Myeloma Society Young Investigator Award: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; OncLive: Honoraria; Survivorship: Honoraria. Lazaryan:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal